- This topic is empty.
-
AuthorPosts
-
2025-11-20 at 2:50 pm #26721
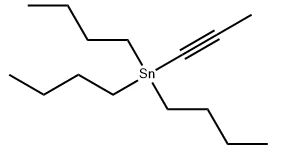
Tributyl(1-propynyl)tin, also known as 1-propynyltributylstannane or tributylpropynyl tin, is a significant organotin compound with diverse applications in organic synthesis, drug development, and materials science. With the CAS number 64099-82-7, this chemical exhibits unique physical and chemical properties that make it indispensable in both laboratory and industrial settings. This article explores the fundamental characteristics, preparation methods, and practical uses of tributyl(1-propynyl)tin. In this blog post, SACH, a high purity organotin compound manufacturing factory, will share the role of Tributyl(1-propynyl)tin in organic synthesis and material science.
Chemical Identity and Basic Properties of Tributylpropynyl Tin
Tributyl(1-propynyl)tin (CAS No.: 64099-82-7) is a colorless liquid with the molecular formula C₁₅H₃₀Sn and a molecular weight of 329.11 g/mol. Its physical characteristics make it easy to handle under controlled conditions:
* Density: 1.082 g/mL at 25 °C
* Boiling Point: 277 °C (literature value)
* Refractive Index: n20/D 1.483
* Flash Point: >230 °F
* Specific Gravity: 1.082
* Hydrolysis Susceptibility: Resistant to reaction with water under neutral conditions
Due to its sensitivity, tributylpropynyl tin is typically stored under an inert gas atmosphere (nitrogen or argon) at temperatures between 2–8 °C to maintain stability. Its resistance to hydrolysis under neutral conditions makes it suitable for use in aqueous-compatible reactions, though caution must be exercised to prevent exposure to reactive conditions.
Synthesis Methods for Tributylpropynyl Stannane
Preparation via Tributyltin Chloride and Sodium Propynyl
One of the primary methods for synthesizing tributyl(1-propynyl)tin (CAS No.: 64099-82-7) involves the reaction between tributyltin chloride and sodium propynyl. Sodium propynyl itself is generated by the reaction of propyne with sodium metal in liquid ammonia. The process proceeds as follows:
1. Combine sodium propynyl with tributyltin chloride under controlled temperature conditions.
2. Allow the reaction to proceed to completion under stirring.
3. Conduct post-reaction treatment to purify the resulting tributylpropynyl tin.
This method is advantageous due to its relatively straightforward protocol and high yield under laboratory conditions.
Coupling Reaction Method
An alternative synthesis route is the catalytic coupling of tributyltin with propyne. This approach often involves the presence of a transition-metal catalyst, which facilitates the formation of the carbon-tin bond efficiently. The reaction mechanism typically includes oxidative addition, transmetallation, and reductive elimination steps, resulting in the desired tributylpropynyl stannane. This method is particularly valuable in industrial-scale production, where catalyst efficiency and reaction control are critical.
Applications in Organic Synthesis
Tributylpropynyl tin (CAS No.: 64099-82-7) plays a pivotal role in modern organic chemistry due to its ability to act as a stannylation reagent. One notable application is in the synthesis of phenyl-substituted pyrimidine dione compounds. The process generally involves:
1. Cycloaddition reactions with ethyl isocyanate to form intermediate structures.
2. Subsequent Stille coupling reactions to introduce the tributyltin group into the molecular framework.
The versatility of tributyl(1-propynyl)tin in forming carbon-carbon bonds makes it a valuable reagent for constructing complex organic molecules. Its stability under neutral conditions ensures that sensitive functional groups remain intact during multi-step synthetic procedures.
Role in Drug Synthesis and PET Imaging
Tributylpropynyl tin also serves as a critical intermediate in pharmaceutical chemistry. For instance, in the synthesis of 18F-labeled triazoquinoline derivatives—ligands used in positron emission tomography (PET)—the compound facilitates the introduction of the tributyltin moiety under palladium-catalyzed coupling conditions.
This step is essential because the tributyltin group provides a handle for subsequent radiolabeling, enabling in vivo imaging of biological processes. The high reactivity and selectivity of tributyl(1-propynyl)tin in these reactions make it an indispensable tool in medicinal chemistry and diagnostic agent development.
Applications in Materials Science
Beyond organic and pharmaceutical synthesis, tributylpropynyl tin has important applications in materials science. Organotin compounds are well-known for their ability to modify polymers, enhance catalyst performance, and introduce functional properties to materials. Specific applications include:
* Polymer Modification: Tributyl(1-propynyl)tin can act as a modifier to adjust polymer properties, improving flexibility, thermal stability, or chemical resistance.
* Catalyst Preparation: It can be incorporated into catalysts used for polymerization or other organic transformations, increasing reaction efficiency and selectivity.
* Advanced Organotin Materials: By introducing the propynyl-tin unit, researchers can create organotin compounds with unique electronic or mechanical properties suitable for specialized industrial applications.
Handling and Storage Considerations
Proper handling of tributylpropynyl tin (CAS No.: 64099-82-7) is crucial due to its chemical activity and sensitivity. Key safety measures include:
* Inert Atmosphere: Storage under nitrogen or argon prevents oxidation and decomposition.
* Temperature Control: Maintain storage temperatures between 2–8 °C to avoid degradation.
* Avoid Strong Acids or Bases: Though hydrolysis under neutral conditions is minimal, exposure to strongly reactive agents may result in unwanted reactions.
Laboratories and industrial facilities working with tributyl(1-propynyl)tin should adhere to standard organotin safety protocols, including personal protective equipment (PPE), fume hoods, and proper waste disposal procedures.
Advantages of Using Tributyl(1-propynyl)tin
The adoption of tributyl(1-propynyl)tin in multiple chemical disciplines is driven by several advantages:
* Chemical Stability: Resistant to neutral aqueous hydrolysis.
* Versatile Reactivity: Effective in carbon-carbon bond-forming reactions such as Stille couplings.
* Functional Diversity: Applicable in pharmaceuticals, polymers, and advanced materials.
* Ease of Handling: Liquid state and moderate density allow precise measurement and transfer in laboratories.
These properties collectively make tributylpropynyl tin a reliable and versatile reagent in modern chemistry.
Conclusion
Tributyl(1-propynyl)tin (CAS No.: 64099-82-7) is a critical organotin compound with wide-ranging applications in organic synthesis, drug development, and materials science. Its unique combination of stability, reactivity, and ease of handling allows chemists to perform complex transformations with high efficiency. From Stille coupling reactions to polymer modification and PET imaging, tributyl(1-propynyl)tin continues to serve as a valuable tool in scientific research and industrial processes. Proper storage under inert gas and controlled temperatures ensures its long-term usability and safety.
By understanding its properties, synthesis methods, and diverse applications, researchers can leverage tributylpropynyl tin to develop new molecules, innovative materials, and cutting-edge diagnostic agents, highlighting its indispensable role in modern chemical science.
-
AuthorPosts
- You must be logged in to reply to this topic.